
Regarding this, what is the 2nd Law of Thermodynamics and give an example? The second law of thermodynamics states that the entropy of any isolated system always increases. What are the 1st 2nd and 3rd laws of thermodynamics? The first law, also known as Law of Conservation of Energy, states that energy cannot be created or destroyed in an isolated system. The First Law of Thermodynamics states that energy cannot be created or destroyed the total quantity of energy in the universe stays the same. The Second Law of Thermodynamics says that processes that involve the transfer or conversion of heat energy are irreversible. The second law also states that the changes in the entropy in the universe can never be negative.Ĭonsequently, what does the 2nd law of thermodynamics mean?
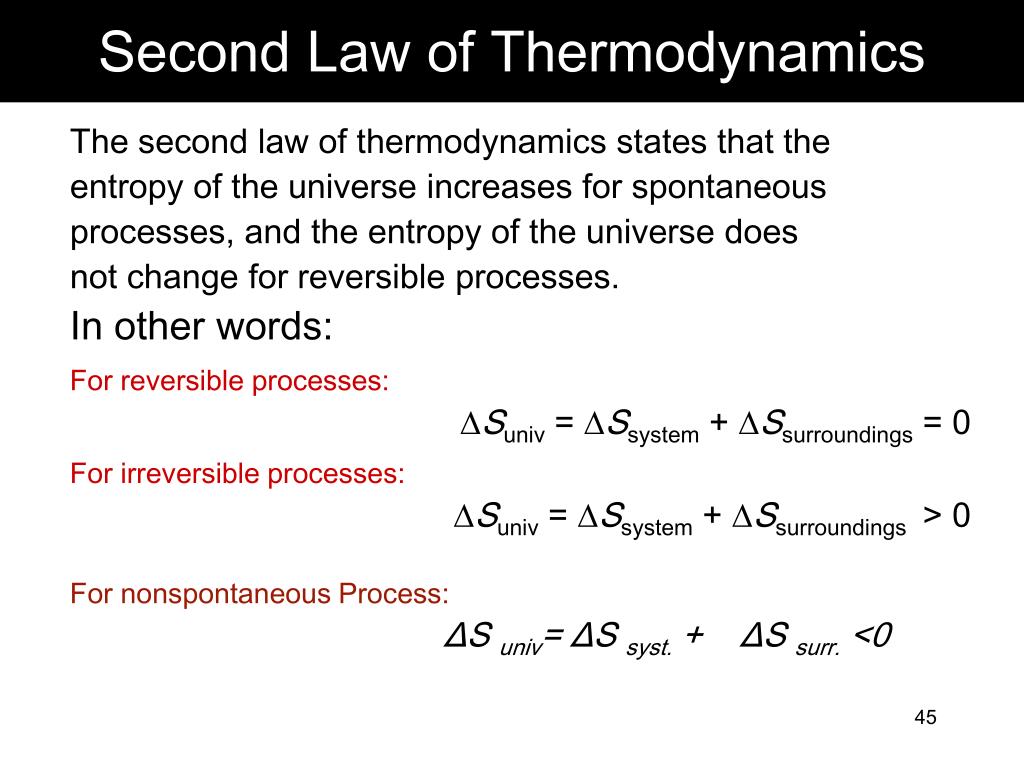

The Second Law of Thermodynamics states that the state of entropy of the entire universe, as an isolated system, will always increase over time.


 0 kommentar(er)
0 kommentar(er)
